NOCTUIDAE - Helicoverpa armigera (Hübner)
Taxonomy
Noctuoidea: Noctuidae: Heliothinae: Helicoverpa armigera (Hübner)
Common names: cotton bollworm, Old World bollworm, scarce bordered straw
Synonyms: Heliothis armigera, H. obsoleta, H. pulverosa, H. uniformis, H. armiger
Prior to the mid-20th century, the name armigera was often used to refer to H. zea in the New World. See the Detailed Information
tab for specifics.
Larval diagnosis (Summary)
- D setae of A1-8 inserted on large conical chalazae, those of A1, A2 or A8 often larger than the rest
- Body color highly variable, but usually with lines and stripes and sometimes a black bar joining the D setae of A1 or A2
- If setal bases are small, then the mandible has a minute tooth on the inner rib and no large retinaculum
- Larvae of H. armigera are difficult to separate from those of other species of Helicoverpa (H. assulta, H. puntigera); see the detailed larval
diagnosis for more information.
- There are no consistent morphological characters that can be used to separate the larvae of H. armigera and H. zea.
Host/origin information
Helicoverpa armigera is highly polyphagous and has been recorded feeding on plants in more than 45 families. Larvae are intercepted on a variety of hosts
from many locations. The origins and hosts listed here are the most common combinations; however, it is possible that larvae may be intercepted on any host originating
from within this species' recorded distribution.
| Origin |
Host(s) |
| India | Tagetes |
| Israel | various |
| Kenya | various |
| Netherlands | Bupleurum, Leucospermum, Ornithogalum, Veronica |
| Zimbabwe | various |
Recorded distribution
Helicoverpa armigera is widely distributed throughout the Old World (Africa, Asia, Europe), Australasia, and Oceania.
In early 2013, H. armigera was first reported from Brazil. This is the first report of H. armigera establishing in the New World. It has since been reported from
the Brazilian states of Mato Grosso, Goias, Bahia, Distrito Federal, Parana, Roraima, and Piaui. In late 2013, it was reported from Paraguay, and in 2014 from the Tucuman Province of
Argentina (Viclos and La Chocha).
Identification authority (Summary)
Larvae of H. armigera are usually intercepted from locations in the Old World. In the New World, H. armigera has been recorded from Brazil (see list of states above),
Paraguay, and Argentina. See the Detailed Information tab for specific identification recommendations for various regions.
Pest characterization
(Based on Cavey 2001, Venette et al. 2003)
- Taxonomy: Medium. Species identification is often difficult.
- Distribution: High. Helicoverpa armigera is not present in the U.S.
- Potential Impact: High. This species is a serious threat to agriculture and the environment.
This ranking characterizes H. armigera as quarantine significant.
NOCTUIDAE - Helicoverpa armigera (Hübner)
Larval diagnosis (Detailed)
Prior to the mid-20th century, the name armigera was often used to refer to H. zea in the New World. Only in 1955 were the two names
considered separate species and H. zea used for the New World populations. Based on Heliothis being considered masculine, the species name
was shortened to "H. armiger" to be in agreement with the gender of the genus. This is not currently followed. Consult King (1994) for a
discussion of these points.
In spite of the pest status of H. armigera, there appears to be few detailed larval morphological studies with complete setal maps of the
mature larva (e.g., Kirkpatrick 1961, Chu et al. 1965, Thakar and Srivastava 1983, Sri et al. 2010). Toguebaye and Couilloud (1982) studied the first
instar of H. amigera in detail.
Various, often conflicted diagnoses, have been published; the major ones are reviewed here organized by geographical regions.
Although complicated, listing the suite of characters used to identify H. armigera will result in a better understanding of morphological
variation and provide backup characters that might be useful in doubtful cases. Neither Weisman (1986) nor Venette et al. (2003) treated larval
identification of H. armigera at United States ports; there is certainly a need to address this topic in some detail.
Hardwick (1965) presented a morphometric key to Helicoverpa larvae based on origins. For Australia, he noted H. armigera usually
had spiracles with dark brown rims and a central light or medium brown area. There was often orange shading around the D and SD pinacula, but no
orange spot. The vertical diameter of SD1 on A7 was equal or less than the diameter of the corresponding spiracle in H. armigera from
Australia (Kirkpatrick 1961). Bejakovich and Dugdale (1998), working in New Zealand, noted H. armigera has three longitudinal bands of
microspines: one on the dorsal midline, one between D2 and L2 and a final one between L1 and L3. In addition, the rim of the spiracle is black
in all instars, the microspines have a broad round base, the cuticle appears "cobblestoned" between the spines and microspines are present
below SD1 on A1-8. Matthews (1999: figs. 415, 416) illustrated the cuticle texture of H. armigera on A1 and A9 with scanning electron
micrographs. He noted late instar H. armigera often have "saddle markings" (enlarged pinacula connected by a black bar) on A1 and A2. These tend to be absent
in H. punctigera (Matthews 1999: plate 21). Another difference is prothoracic setae behind the head. These tend to be pale in
H. armigera but dark in H. puntigera. Hosts can also provide a clue. Helicoverpa punctigera is not as common on members
of the grass family as is H. armigera (Department of Primary Industries and Fisheries 2005).
Chu et al. (1965), studying Helicoverpa in China, noted that H. armigera and H. assulta both have a small inner mandibular tooth. Helicoverpa armigera
has cone shaped pinacula on A1 and A8 and more spines near the dorsal line on A1 and A8 than H. assulta. The spines of H. armigera are pointed, easy to see
"inside the legs" and the head has P1 slightly higher than AF2. Gardner (1946) put H. armigera in his A IV group noting that these species have a trisetose
SV group on A2, a bisetose SV group on A7, SD1 on A8 directly above the spiracle and microspines becoming hairlike ventrally. Gardner (1946) did not find
an inner tooth on the mandible of H. armigera. Yoshimatsu (2002), working in Japan, illustrated the mandibles of both H. armigera and H. assulta.
They were nearly identical but H. armigera was said to have microspines on the anal shield that are lacking in H. assulta. In addition, the diameter
of the SD1 pinacula on A7 is about equal to the spiracle length in H. armigera. The SD1 pinacula on A7 in H. assulta is much larger, about 1.4-2
times the length of the spiracle. There are also minor differences in spiracle sizes on A7 compared to A8 between these species.
Hardwick (1965) used measurements and ratios to separate H. armigera from H. assulta in Africa without other morphological details.
Ahola and Silvonen (2005) gave a detailed description of the mouthparts of H. armigera, but no keys to related species in northern Europe.
Beck (1999: 291-293) did provide a key to European Heliothinae. He illustrated the chaetotaxy of the first abdominal segment, noted the
D pinacula of A1 and A8 are joined by a black bar and that the D pinacula of A1 are relatively large and closely spaced (Beck 1999: 501b, Beck 2000: 174).
He illustrated the mandible of H. armigera with a small inner tooth (Beck 1999: 512c). Sannino et al. (1993) included H. armigera
in his study of Lepidoptera associated with tobacco. He called attention to the sinuate striations and well developed pinacula in H. armigera.
Given the documented larval variation in North American species of Chloridea, Heliothis, and Helicoverpa, we have little or
no confidence in morphological identification of larval H. armigera in some parts of its range. Neunzig (1969: figs 9-12) showed that the
pinacula height of both H. zea and C. virescens varies according to age within an instar. As a larva grows, the cuticle tightens
which tends to stretch and shrink the pinacula. Obviously, it becomes hard to trust pinacula size as a key character, although several authors
cite conical pinacula as a feature of H. armigera. Another problem is mandible wear. Chloridea virescens can have a well developed
inner tooth or just a scar (Neunzig 1969: figs 6, 7). This may explain why some authors see a tooth on H. armigera while others do not.
Unfortunately, the quarantine significance of H. armigera forces us to evaluate conflicting literature and attempt a diagnosis. In
New Zealand, the black spiracles and three bands of microspines will separate H. armigera from H. puntigera (with brown spiracles)
and H. assulta (with microspines not in obvious bands) (Bejakovich and Dugdale 1998: 10, 11). Kirkpatrick (1961) was unable to separate
H. armigera from H. punctigera in Australia, and given doubt about the spiracular color of H. armigera being black or
brown in areas outside of New Zealand, it is better to stop at genus for interceptions in Australia. Color characters (saddle markings, etc.)
will be a clue but are not definitive. Yamaskaki et al (2009: Fig. 1) illustrated four color forms of H. armigera: completely green,
green with stripes, brown and black. This helps document at least some of the expected variation for this pest.
For European interceptions, when present, large conical pinacula will separate H. armigera from related genera of Heliothinae,
but not all specimens are expected to show this character. Perhaps scattered spines on the distal region of the hypopharyngeal complex
(Ahola and Silvonen 2005: fig. 1106) is the most distinctive mouthpart character for H. armigera in northern Europe. The final
problem is separating H. armigera from H. assulta. Helicoverpa assulta is normally associated only with only
Solanaceae (but see Mathews 1999: 117, 118) whereas H. armigera is polyphagous. Differences in cuticular texture will separate
the two species on solanaceous hosts. Sannino et al (1996) compared H. peltigera to H. armigera and noted major differences
in the larval mandibles.
Identification authority (Detailed)
Helicoverpa armigera was first reported as established in Brazil in early 2013 in the states of Goias, Bahia, and Mato Grosso (Czepak et al. 2013). It has
since been reported from the Distrito Federal, the southern state of Parana (Specht et al. 2013, Tay et al. 2013), and also Roraima and Piaui (Mastrangelo et al. 2014).
Individuals of H. armigera have also been confirmed from Paraguay and the Tucuman Province in Argentina (Murua et al. 2014).
This is the first instance of this important pest establishing in the New World. Because we have not identified any morphological characters to reliably separate
H. zea from H. armigera, interceptions from many locations in South America will require molecular methods. There is also occasional evidence of Old World
cargo or produce being shipped through Mexico to the United States. This is another potential pathway for introduction of H. armigera into the New World.
A second problem area is Australia and New Zealand. Do not identify H. armigera to species in Australia and do so in New Zealand only with caution using
well preserved mature larva. Helicoverpa puntigera is restricted to Australia, or occasionally New Zealand where it does not overwinter (Bejakovich and Dugdale 1998: 52),
and therefore it is not a consideration when identifying H. armigera from other regions.
For quarantine purposes, it may be best to assume H. assulta eats only Solanaceae until more records from other plant families
are published from regions outside of Australia. Therefore, we can assume Helicoverpa interceptions from non-solanaceous plants
are more likely to be H. armigera than H. assulta. Body color is another clue. Hardwick (1965:28) stated that H. assulta
is primarily a green larva whereas H. armigera tends to be darkly colored. This difference between the two species was illustrated
by Li et al. (2013) along with other morphological characters needing confirmation (crochet pattern) or clarification (prothoracic chaetotaxy).
Therefore, green larvae on Solanaceae from the Old World need to be examined carefully as H. assulta suspects instead of rapidly confirming
them as H. armigera.
Identification of H. armigera from Europe and Northern Africa is relatively straightforward. Heliothis nubigera is the only species almost colored
like H. armigera (Beck 1999-2000, Ahola and Silvonen 2005) from this region. The literature is good and sibling
species like H. assulta do not occur in those regions. Netherlands vegetables are assumed to be grown in country, other host are of
uncertain origin and therefore it is best not to attempt a species identification for Helicoverpa from this pathway. Sibling species
of Helicoverpa in Central Africa prevent recognition of H. armigera in that part of the world.
NOTE: Due to the continuing spread of H. armigera in South America, exercise caution when attempting identifications from that continent. There are no morphological
characters to separate the larvae of H. armigera from H. zea. When in doubt, default to "Helicoverpa sp." instead of attempting a species-level ID. The following
keys may not reflect the most recent H. armigera distribution in South America.
NOCTUIDAE - Helicoverpa armigera (Hübner)
Because larvae of H. armigera are difficult to identify to species, many are likely listed in PestID under " Helicoverpa spp."
Origin records
Helicoverpa armigera has been intercepted from the following locations:
Albania, Bosnia and Herzegovina, Bulgaria, Burkina Faso, Cape Verde, China, Egypt, Eritrea, France, Gambia, Germany, Ghana, Guatemala, Hong Kong, India,
Iraq, Israel, Italy, Japan, Jordan, Kenya, Lebanon, Malaysia, Mali, Morocco, Mozambique, Netherlands, New Zealand, Nigeria, Pakistan, Palestinian Territory,
Philippines, Portugal, Senegal, South Africa, South Korea, Spain, Tanzania, Thailand, Togo, Trinidad and Tobago, Tunisia, Turkey, Ukraine, United Arab Emirates,
United Kingdom of Great Britain and N. Ireland, Yugoslavia, Zimbabwe
Records from Guatemala needs confirmation and likely represent misidentifications.
Host records
Helicoverpa armigera has been intercepted on the following hosts:
Abelmoschus esculentus, Achillea, Allium, Amaranthus, Ammi, Anemone coronaria, Anemone, Anethum,
Anigozanthos, Antirrhinum, Artemisia, Asclepias, Asclepias tuberosa, Asparagus, Aster, Astilbe,
Astrantia, Bellis, Bouvardia, Brassica oleracea, Brassica oleracea var. acephala, Brassica rapa,
Brassica, Brunia, Bupleurum griffithii, Bupleurum, Cajanus cajan, Calendula, Capsicum annuum, Capsicum,
Carthamus, Celosia, Cestrum, Chamelaucium, Chrysanthemum, Cicer arietinum, Cicer, Cichorium intybus,
Citrus, Clematis, Coriandrum sativum, Cosmos, Craspedia, Cucurbita, Cucurbitaceae, Cynara scolymus,
Dahlia, Delphinium, Dendrobium, Dianthus, Echinops, Eryngium, Eustoma grandiflorum, Eustoma, Fabaceae,
Fragaria, Gentiana, Gerbera, Gladiolus, Gomphrena, Grevillea, Gypsophila, Helianthus, Hibiscus,
Hydrangea, Hypericum, Jasminum sambac, Jasminum, Lablab purpureus, Lathyrus sativus, Leucadendron,
Leucospermum cordifolium, Leucospermum, Limonium, Lippia graveolens, Lippia, Lisianthus, Lysianthus,
Lysimachia, Malus, Malvaceae, Matricaria, Mentha longifolia, Mentha, Minthostachys, Moluccella, Musa,
Nerium, Ocimum basilicum, Ocimum, Oleaceae, Oncidium, Origanum majorana, Origanum, Origanum vulgare,
Ornithogalum arabicum, Ornithogalum, Oxypetalum, Phaseolus coccineus, Phaseolus, Pisum sativum, Pisum,
Polianthes tuberosa, Polyanthus, Protea, Ranunculus, Rosa, Rosmarinus officinalis, Rosmarinus,
Rudbeckia, Salvia officinalis, Salvia, Satureja hortensis, Satureja, Scabiosa, Setaria italica, Solanaceae,
Solanum aethiopicum, Solanum melongena, Solanum, Statice, Tagetes erecta, Tagetes, Tetrapleura,
Thymus citriodorus, Thymus, Thymus vulgaris, Trachelium, Tuberosa, Tulipa, Vernonia amygdalina,
Veronica, Zea mays
NOCTUIDAE - Helicoverpa armigera (Hübner)
Setal map
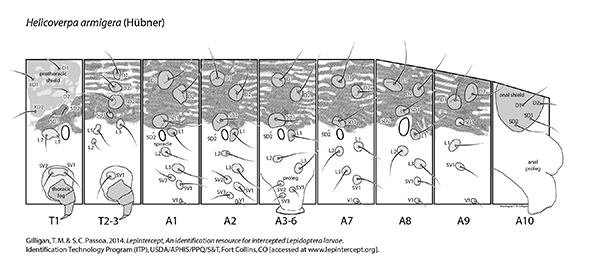 Helicoverpa armigera setal map Helicoverpa armigera setal map
<< Previous fact sheet Next fact sheet >>
 Fig. 1: Late instar larva, lateral view
Fig. 1: Late instar larva, lateral view |
 Fig. 2: Late instar larva, dorsal view
Fig. 2: Late instar larva, dorsal view |
 Fig. 3: Late instar larva, lateral view
Fig. 3: Late instar larva, lateral view |
 Fig. 4: Late instar larva, dorsal view
Fig. 4: Late instar larva, dorsal view |
 Fig. 5: Mid- to late instar larva, lateral view
Fig. 5: Mid- to late instar larva, lateral view |
 Fig. 6: Mid- to late instar larva, dorsal view
Fig. 6: Mid- to late instar larva, dorsal view |
 Fig. 7: Mid-instar larva, lateral view
Fig. 7: Mid-instar larva, lateral view |
 Fig. 8: Early instar larva, lateral view
Fig. 8: Early instar larva, lateral view |
 Fig. 9: Early instar larva, lateral view
Fig. 9: Early instar larva, lateral view |

Fig. 10: Mid-instar |

Fig. 11: Head, thorax |
 Fig. 12: Conical pinacula on A1
Fig. 12: Pinacula on A1 |

Fig. 13: Crochets |
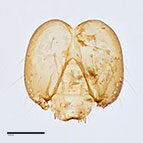
Fig. 14: Head (cleared) |
 Fig. 15: Hypopharyngeal complex, lateral view
Fig. 15: Hypo. complex |
 Fig. 16: Hypopharyngeal complex, dorsal view
Fig. 16: Hypopharyngeal complex, dorsal view |

Fig. 17: Mandible |

Fig. 18: Mandible |
|
|
|
|
|