NOCTUIDAE - Copitarsia
Taxonomy
Noctuoidea: Noctuidae: Cuculliinae: Copitarsia
Larval diagnosis (Summary)
- T2-3 with both SD1 and SD2 connected to a minute tonofibrillary platelet by a sclerotized bar
- Mandible without a retinaculum
- Spinneret rounded or with a medial depression
- Labial palpi with last segment much shorter than basal segment
- Late instar larvae with white body setae, paired dorsal dashes or triangles, lateral red spots, or no markings
- Early instar larvae with a mottled head, dark setae, reduced prolegs on A3 and A4, and a green dorsum faintly striped with white
Host/origin information
Most interceptions of Copitarsia originate from Colombia (>60%) or Mexico (>25%). Other countries and associated hosts are listed below:
| Origin |
Host(s) |
| Colombia | Alstroemeria, Aster, Callistephus, Chrysanthemum, Dianthus, Limonium,
Mentha, Moluccella, Solidago |
| Mexico | Alstroemeria, Brassica, Chenopodium, Coriandrum |
| Chile | Asparagus |
| Ecuador | Alstroemeria, Helianthus, Moluccella |
Recorded distribution
Species of Copitarsia occur from central Mexico along the western edge of Central and South America to southern Argentina (Gould et al. 2013). There are also interception
records from the Caribbean. The genus is not reported as established outside of the New World.
Identification authority (Summary)
Copitarsia larvae can be recognized to genus using morphology. In a few cases, host and orgin will allow a tentative species name.
Copitarsia are more commonly intercepted from Mexico and South America than most of Central America except Guatemala. It does not occur in the Old World.
Pest characterization
- Taxonomy: Medium. Most identifications are to genus.
- Distribution: High. Copitarsia do not occur in the U.S.
- Potential Impact: High. Several Copitarsia are pests.
This ranking characterizes Copitarsia as quarantine significant for the U.S.
NOCTUIDAE - Copitarsia
Larval diagnosis (Detailed)
There are from six to 25 species in Copitarsia depending on the taxonomic authority, but only a few of these are economic
pests (Gould et al. 2013). Consult Pogue (2014) for a critical review of nomenclature in the Copitarsia decolora complex,
including host plants and some larval descriptions. Most of the species of concern to APHIS are treated in his publication.
Additional descriptions are given by Angulo et al. (1985), Arce de Hamity and Neder de Roman (1993), Angulo and Olivares (2005),
Angulo et al. (2006), and Zuniger et al. (2006). Color illustrations of larval Copitarsia were published by CESAVEP (2002),
Angulo et al. (2006), Passoa (2007), Pogue [2010], and Gould et al. (2013).
Typically, late instars of Copitarsia are recognized by the short last segment of the labial palpi, a median depression at
the apex of the spinneret and the presence of a minute sclerotized bar connecting both the SD1 and SD2 setal bases to a ventral
tonofibrillary platelet on the mesothorax and metathorax (Weisman 1986). In addition, the mandible always lacks a retinaculum,
the mature larva has white body setae and the early instar larva has a mottled head, dark body setae, and a green dorsum having
faint white stripes (Riley unpublished, Weisman 1986, Passoa 2007). The first three instars have reduced prolegs on A3 and A4
and move in a looping fashion (semi-loopers); later instars have abdominal prolegs of equal size allowing normal movement without
looping (Arce de Hamity and Neder de Roman 1993: 32).
However, both the spinneret morphology and body coloration of Copitarsia are variable. Not all species have an equal
indentation at the apex of the spinneret. Copitarsia gibberosa has deep indentation, giving the impression of two lobes
(Pogue 2014). The spinneret of C. corruda and C. decolora are both broadly rounded, but C. decolora differs from the
former species in having a slight median notch (Pogue and Simmons 2008). Neither C. naenoides (Angulo et al. 2005) nor
C. incommoda (Pogue 2011) have a median indentation on the spinneret.
Body coloration is also variable both in markings and ground color. Copitarsia decolora from Mexico, previously known as
C. consueta, has a green or brown color form, each with or without large dorsal triangular markings on the last few abdominal
segments and sometimes with red markings near the abdominal spiracles (CESAVEP 2002). Pogue (2011) noted green and brown color
forms also occur in C. corruda and that the dorsal markings are like dashes in C. incommoda. Passoa (2007) illustrated
Copitarsia larvae showing a green morph with red near the spiracles, brown morphs in two shades and an early instar larva
with Spodoptera exigua-like coloration.
Given the similar coloration, it is easy to confuse early instar Copitarsia and Spodoptera exigua. However, Copitarsia
never has a black mesothoracic spot typical of S. exigua. In addition, the head of Copitarsia, although variable in pattern,
is often more speckled than S. exigua (compare Wagner et al. 2011 to Pogue [2010]). The spinneret is pointed in S. exigua
but blunt in Copitarsia. Other differences are given by Riley (unpublished). Copitarsia could also be confused with
Peridroma saucia; characters to separate the two are in Weisman (1986).
In some situations, the ability to recognize Copitarsia eggs can be important, especially on asparagus.
Gonzales-Bustamante (2008), Angulo and Olivares (2009), and Andaur-Arenas and Olivares (2009) have illustrated
Copitarsia eggs in enough detail to recognize the genus if there are few other possibilities and the fauna is
well documented.
Identification authority (Detailed)
Copitarsia larvae can be recognized to genus using morphology. In a few cases, host and orgin will allow a tentative
species name. As a rule, Copitarsia are more commonly intercepted from Mexico and South America than most of Central America
except Guatemala (Pogue 2014). It does not occur in the Old World. Pogue (2014) summarized exact distributions for
the economically important species.
Because of misidentifications in the literature, hostplants are of little value (Pogue 2014). Copitarsia decolora
is the only species in Mexico and Central America, except for one anomalous record of C. corruda in Mexico (Pogue 2014,
Pogue and Simmons 2008). For now it seems accurate to name all Mexican and Central American specimens C. decolora based on
origin. However, that could change if further study shows C. corruda is established in the region.
Specimens from Peru on asparagus, if the morphology and coloration fit Pogue and Simmons (2008), can be called
C. corruda.
Specimens of Copitarsia with segmental abdominal dashes from western South America could be C. incommoda. Other
interceptions of Copitarsia on other hosts are best left at genus. The shape of the spinneret is helpful, but too many
species of Copitarsia have undescribed larvae for species determinations in most of South America.
The genus Copitarsia does not have any widely accepted English common names. The Spanish common name for C. decolora is
"gusano del corazon de la col" (Saunders et al. 1983). Although widely distributed in Latin America, C. decolora is well known in
Mexico. Therefore, we can suggest Mexican cabbage heartworm or Mexican cabbage head caterpillar for this species.
NOCTUIDAE - Copitarsia
Origin records
Copitarsia have been intercepted from the following locations:
Argentina, Aruba, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, El Salvador, Guatemala, Guyana,
Haiti, Honduras, Israel*, Jamaica, Mexico, Netherlands*, Nicaragua, Panama, Peru, Puerto Rico, Venezuela
Copitarsia do not occur outside of the New World. Thus, interception records from Europe or the Middle East likely represent
transshipments or mixing of commodities (usually cut flowers) from South America (denoted with an *).
Host records
Copitarsia have been intercepted on the following hosts:
Abelmoschus esculentus, Achillea millefolium, Achillea sp., Aconitum sp., Agapanthus sp., Ajuga sp., Allium ampeloprasum,
Allium cepa, Allium fistulosum, Allium schoenoprasum, Allium sp., Aloe vera, Alstroemeria aurantiaca, Alstroemeria sp.,
Amaranthus sp., Amaryllis sp., Ammi majus, Ammi sp., Ammi visnaga, Ananas comosus, Anemone sp., Anethum graveolens,
Anethum sp., Anigozanthos sp., Anigozanthus sp., Annona cherimola, Anthemis sp., Anthurium sp., Antirrhinum majus,
Antirrhinum sp., Apium graveolens, Apium graveolens var. dulce, Apium sp., Arachniodes sp., Artemisia dracunculus,
Artemisia sp., Asparagus officinalis, Asparagus sp., Aster sp., Astilbe sp., Basilicum sp., Beta sp.,
Beta vulgaris var. cicla, Beta vulgaris var. vulgaris, Bouvardia sp., Brassia sp., Brassica chinensis,
Brassica napus, Brassica oleracea, Brassica oleracea var. alboglabra, Brassica oleracea var. botrytis, Brassica oleracea var. capitata,
Brassica oleracea var. italica, Brassica pekinensis, Brassica rapa ssp. chinensis, Brassica rapa ssp. pekinensis,
Brassica rapa var. parachinensis, Brassica sp., Bupleurum sp., Cactaceae, Callistephus chinensis, Callistephus sp.,
Calluna vulgaris, Campanula cochlearifolia, Campanula sp., Capsicum sp., Carthamus sp., Celosia argentea var. cristata,
Celosia sp., Chamaemelum nobile, Chamaemelum sp., Chamomilla sp., Chenopodium album, Chenopodium ambrosioides,
Chenopodium berlandieri ssp. nuttalliae, Chenopodium sp., Chrysanthemum sp.,
Chrysanthemum x morifolium, Cicer arietinum, Cichorium endivia, Cichorium intybus, Citrus aurantiifolia*, Codiaeum sp.,
Colocasia esculenta, Consolida sp., Coriandrum sativum, Coriandrum sp., Crocosmia sp., Cupressaceae, Cynara scolymus,
Dahlia sp., Daucus sp., Delphinium sp., Dendranthema sp., Dendrathema sp., Dianthus barbatus, Dianthus caryophyllus,
Dianthus sp., Digitalis sp., Dracaena marginata, Dysphania ambrosioides, Eruca sativa, Eryngium foetidum, Eryngium sp.,
Eucalyptus sp., Euphorbia sp., Euphorbiaceae, Eustoma sp., Fragaria sp., Freesia sp., Gerbera sp., Gladiolus sp.,
Glycine max, Godethia sp., Godetia sp., Grevillea sp., Gypsophila elegans, Gypsophila paniculata, Gypsophila sp.,
Helianthus annuus, Helianthus sp., Helichrysum sp., Heliconia sp., Hippeastrum sp., Hydrangea sp., Hypericum sp.,
Iris sp., Lactuca sativa, Lactuca sp., Lathyrus odoratus, Laurus sp., Leucadendron sp., Leucaena sp., Liatris sp.,
Liatris spicata, Lilium sp., Limonium sinuatum, Limonium sp., Lippia sp., Lisianthus sp., Lysimachia sp., Majorana sp.,
Malus domestica, Marjorana hortensis, Mathiola sp., Matthiola incana, Matthiola sp., Mentha arvensis, Mentha piperita,
Mentha sp., Minthostachys sp., Moluccella laevis, Moluccella sp.,
Ocimum basilicum, Ocimum sp., Opuntia sp., Origanum majorana, Origanum sp., Origanum vulgare,
Ornithogalum sp., Paeonia sp., Persea americana*, Petroselinum crispum, Petroselinum sp., Phaseolus sp., Phaseolus vulgaris,
Philodendron sp., Phlox sp., Physalis ixocarpa, Physalis philadelphica, Physalis pubescens, Physalis sp., Pinus sp.*,
Pisum sativum, Pisum sativum var. macrocarpon, Pisum sp., Poeaceae*, Polianthes sp., Polianthes tuberosa,
Porophyllum sp., Portulaca oleracea, Portulaca sp., Protea sp., Prunus armeniaca*, Prunus domestica*, Prunus persica*,
Prunus persica var. nucipersica*, Pyrus pyrifolia*, Ranunculus sp., Raphanus sativus, Raphanus sp., Rosa sp.,
Rosmarinus officinalis, Rubus idaeus, Rubus sp., Ruscus sp., Salvia officinalis, Salvia sp., Sechium edule,
Solanum melongena, Solanum sp., Solidago canadensis, Solidago sp., Solidaster sp., Spinacia oleracea, Statice sp.,
Strelitzia sp., Suaeda sp., Tagetes sp., Thymelaea hirsuta, Thymus sp., Thymus vulgaris, Trachelium sp., Tulipa sp.,
Vaccinium sp., Veronica sp., Vicia faba, Vigna sp., Zantedeschia aethiopica, Zantedeschia sp., Zea mays
Records from citrus, avocado, grasses, tree fruits, and pine need confirmation (denoted with an *).
NOCTUIDAE - Copitarsia
Setal map
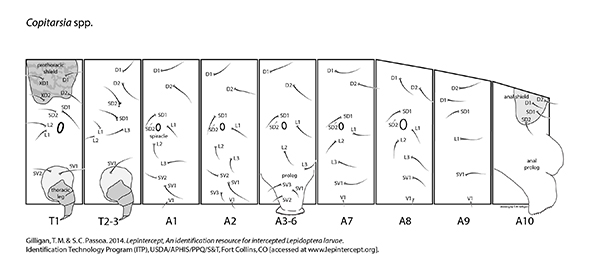
Copitarsia setal map
<< Previous fact sheet Next fact sheet >>
 Fig. 1: Late instar, lateral view
Fig. 1: Late instar, lateral view |
 Fig. 2: Mid-instar, lateral view
Fig. 2: Mid-instar, lateral view |
 Fig. 3: Early instar, lateral view
Fig. 3: Early instar, lateral view |
 Fig. 4: Late instar head and T1 shield, dorsal view
Fig. 4: Head, T1 shield |
 Fig. 5: Mid- to early instar head and T1 shield, dorsal view
Fig. 5: Head, T1 shield |
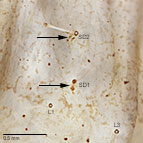 Fig. 6: T2-3 with both SD1 and SD2 connected to a minute tonofibrillary platelet by a sclerotized bar
Fig. 6: SD1-2 on T2-3 |

Fig. 7: Crochets |
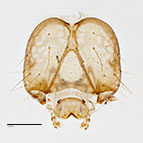
Fig. 8: Head |
 Fig. 9: Hypopharyngeal complex, lateral view
Fig. 9: Hypo. complex |

Fig. 10: Mandible |
|
|
|
|
|
|