NOCTUIDAE - Helicoverpa zea (Boddie) *Non-Rep*
Taxonomy
Noctuoidea: Noctuidae: Heliothinae: Helicoverpa zea (Boddie)
Common names: corn earworm, tomato fruitworm, cotton bollworm
Synonyms: Heliothis zea, H. umbrosus, H. ochracea, H. stombleri
Prior to the mid-20th century, the name armigera was often used to refer to H. zea in the New World.
Larval diagnosis (Summary)
- Mandible without retinaculum
- No microspines on middle to top of the body pinacula
- Body color and patterns variable
- Larvae of H. zea can difficult to separate from those of H. phloxiphaga in Mexico or sibling species in South America; see the detailed
larval diagnosis for more information
- There are no consistent morphological characters that can be used to separate the larvae of H. armigera and H. zea
Host/origin information
The majority of interception records (>70%) are from Mexico, with nearly half of those associated with corn (Zea mays). Larvae of H. zea are highly polyphagous and
interceptions have been recorded from more than 180 types of plants. Records in PestID from locations outside of the Americas or the Caribbean could represent H. zea or any of several
sibling species with poorly known larvae.
| Origin |
Host(s) |
| Dominican Republic | Capsicum |
| Ecuador | various |
| Guatemala | various |
| Mexico | Capsicum, Cicer, Lactuca, Ocimum, Phaseolus, Physalis, Zea mays |
| Nicaragua | various |
| Peru | Psium, Zea mays |
| Trinidad and Tobago | Capsicum |
Recorded distribution
Helicoverpa zea is widely distributed throughout the Americas. It is present in North, Central, and South America, the Caribbean, and Hawaii. It has also been recorded from
other Pacific Islands (populations which likely did not establish) (Hardwick 1965).
Identification authority (Summary)
Only mid- to late instar larvae can be identified using the mandible and lack of microspines on the pinacula; early instars should be identified only to subfamily. Although
H. zea is only recorded from the New World, the presence of other Helicoverpa can make a species-level identification difficult. Consult the Detailed Information tab for information on other species
present in South America. The discovery of H. armigera in Brazil, Paraguay, and Argentina makes species-level identifications from those countries impossible using only morphology.
Pest characterization
(Based on Cavey 2001, Hardwick 1965)
- Taxonomy: High. Species identification is often possible.
- Distribution: Low. Helicoverpa zea occurs in the U.S.
- Potential Impact: High. Helicoverpa zea is a pest species.
This ranking characterizes H. zea as not quarantine significant for the U.S.
NOCTUIDAE - Helicoverpa zea (Boddie) *Non-Rep*
Larval diagnosis (Detailed)
The larva of the corn earworm, Helicoverpa zea, was at least partially described by many authors including Garman (1920), Crumb (1956),
Okumura (1961), Peterson (1962), Hardwick (1965), Neunzig (1969), Pastrana and Henandez (1979), Godfrey (1987), and Hardwick (1996). Several
larval color patterns were photographed by Hardwick (1996), Cranshaw (2004), and Wagner et al. (2011). Brazzel et al. (1953) illustrated
variation in the mandibles of H. zea. Early literature was reviewed Kogan et al. (1978). The Heliothinae guide (below) provides keys to separate H. zea
from other quarantine species of Heliothinae.
Larval variation in the Chloridea/Heliothis/Helicoverpa complex can complicate identifications. Although simplified keys work for most
situations in the United States, APHIS intercepts so many individuals that exceptions are common and have to be discussed.
Typically, H. zea is recognized by having no retinaculum on the mandible and a lack of microspines on the body pinacula (e.g., Godfrey 1987).
These characters separate H. zea from most Chloridea virescens. The mandible of H. zea normally lacks a retinaculum, although a small tooth may be
present. A retinaculum, usually well developed in C. virescens, may be reduced to a thin ridge or "scar." Each of these cases was illustrated
by Brazzel et al. (1953) and Neunzig (1969: 11). A small number of microspines may be found on the body pinacula of H. zea in the fifth and
sixth instars, but only around the edges (Brazzel et al. 1953: 16). The microspines of C. virescens tend to reach at least the middle of the
body pinacula on many segments (see C. virescens fact sheet). Boyer et al. (1977) suggested the most accurate way to evaluation the microspine
character was to use the dorsal pinacula on A8.
Typically, Heliothis phloxiphaga has conical pinacula all over the body. Conical pinacula in H. zea, if present, are only on A1, A2, and A8.
Some specimens of H. phloxiphaga have flat pinacula (Hardwick 1996: N7, N8); these can be confused with H. zea. Unlike H. zea, H. phloxiphaga
sometimes has dark arcs on the head (Crumb 1926) or pinacula ringed with white (Lange and Michelbacher 1937).
Normally, H. zea does not feed on Physalis (Godfrey 1987), while Chloridea subflexa is a Physalis specialist. However, Robinson et al. (2002)
recorded H. zea from a species of Physalis, thus morphological characters should be carefully checked to separate larvae found on Physalis.
Peterson (1962: L36) illustrated differences in the spine pattern of A4 which can be used to separate C. subflexa from H. zea. In addition,
the SD2 seta is surrounded by a sclerotized area in C. subflexa but not in H. zea (Peterson 1962: L36, Wagner et al. 2011).
The key by Hardwick (1965) is useful but not practical for APHIS. The first couplet lacks entries for Europe, Central America and the
Caribbean. Granted, these areas can be treated with other existing keys. A bigger problem with his morphometric study is that the cuticle
stretches between instars causing the pinacula to shrink (Neunzig 1969:11). It is unknown if this affects the setal ratios that Hardwick
(1965) used to recognize species, although he claimed a high success rate for the key.
Identification authority (Detailed)
Helicoverpa zea is highly polyphagous and widely distributed, but there are no records outside of the Americas, Hawaii and scattered collections on other
Pacific Islands that may not have established (Hardwick 1965). The above diagnoses will allow species identifications from North America, Central America and
the Caribbean.
It should be noted that H. phloxiphaga occurs only as far south as Mexico, and is quite rare in United States port interceptions. The other species
(H. zea, C. virescens, and C. subflexa) are commonly intercepted.
Only the mid to last instars of H. zea can be identified by the mandible and cuticle microspines. First and second instars should be left at subfamily or consult
Neunzig (1969) if there is a reason to make an identification of early instars of H. zea and C. virescens. For the quarantine decisions in the United States, both
have the same action status, and this effort is usually not justified.
Identification of H. zea from South America is often not possible. One problem is the introduction of H. armigera to Brazil (Czepak et al. 2013), Paraguay,
and Argentina (Murua et al. 2014). No morphological characters are yet known to separate the larvae of H. armigera from H. zea; thus we can only go to
genus Helicoverpa from these locations in South America.
Currently (June, 2014), it is possible to identify H. zea from Ecuador, Colombia, and Venezuela. There are sibling species of the zea complex in Peru and other
parts of South America, especially H. gelotopoeon. For Peru and the rest of South America, it is best to say "Helicoverpa sp." Consult the Heliothinae guide
(below) for details.
For Hawaii, if the interception is from corn, and the larva has conical pinacula on A1, A2 and A8, then it can be called H. zea (Beardsley 1982).
Helicoverpa hawaiiensis (Beardsley 1982) and some H. zea (SPIC) have flat pinacula. Chloridea virescens also occurs in Hawaii (Beardsley 1982); it may
be identified by the characters given above.
NOTE: Due to the continuing spread of H. armigera in South America, exercise caution when attempting identifications from that continent. There are no morphological
characters to separate the larvae of H. armigera from H. zea. When in doubt, default to "Helicoverpa sp." instead of attempting a species-level ID. The following
keys may not reflect the most recent H. armigera distribution in South America.
NOCTUIDAE - Helicoverpa zea (Boddie) *Non-Rep*
Origin records
Helicoverpa zea has been intercepted from the following locations:
Argentina, Barbados, Belize, Bolivia, Brazil, British Virgin Islands, Canada, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic,
Ecuador, El Salvador, Guatemala, Guyana, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Peru, Puerto Rico, St. Lucia,
Trinidad and Tobago, US Virgin Islands, Venezuela
Locations from outside of the Americas or the Caribbean have been omitted as these likely represent misidentifications.
Host records
Helicoverpa zea has been intercepted on the following hosts:
Abelmoschus esculentus, Abelmoschus sp., Allium fistulosum, Allium porrum, Allium sp., Aloe vera, Alstroemeria sp., Amaranthus caudatus,
Amaranthus sp., Ananas comosus, Anethum graveolens, Annona cherimola, Antirrhinum majus, Antirrhinum sp., Apiaceae, Apium graveolens,
Apium graveolens var. dulce, Apium sp., Artemisia dracunculus, Artocarpus altilis, Aster sp., Basilicum sp.,
Beta vulgaris var. cicla, Brassica chinensis, Brassica oleracea, Brassica oleracea var. botrytis, Brassica oleracea var. capitata,
Brassica pekinensis, Brassica rapa, Brassica rapa ssp. chinensis, Brassica rapa ssp. pekinensis, Brassica sp., Cajanus cajan, Calendula officinalis,
Calendula sp., Campanula sp., Capsicum annuum, Capsicum frutescens, Capsicum sinense, Capsicum sp., Carthamus sp., Chamaedorea sp.,
Chamomilla sp., Chenopodium album, Chenopodium berlandieri ssp nuttalliae, Chenopodium sp., Chichorium sp., Chicorum sp., Chrysanthemum sp.,
Cicer arietinum, Cicer sp., Cichorium intybus, Cichorium sp., Citrullus lanatus, Clematis sp., Colocasia sp., Corchorus capsularis,
Coriandrum sativum, Cucumis sativus, Cucurbita maxima, Cucurbita pepo, Cucurbita sp., Cyamopsis tetragonoloba, Cynara cardunculus, Cynara scolymus,
Daucus carota, Delphinium elatum, Delphinium sp., Dianthus caryophyllus, Dianthus sp., Equisetum sp., Eucalyptus sp., Fabaceae, Fragaria sp.,
Gerbera sp., Gladiolus sp., Gypsophila paniculata, Gypsophila sp., Helianthus annuus, Helianthus sp., Juglans sp., Lablab sp.,
Lactuca sativa, Lactuca sativa var. capitata, Lactuca sp., Lagenaria siceraria, Lagenaria sp., Leucadendron sp., Leucospermum sp., Limonium perezii,
Limonium sp., Lippia sp., Luffa acutangula, Lycopersicon lycopersicum, Malus domestica, Mangifera indica, Matricaria recutita, Matricaria sp.,
Matthiola incana, Medicago sativa, Mentha arvensis, Mentha longifolia, Mentha piperita, Mentha sp., Mimosoideae,
Moluccella sp., Momordica charantia, Momordica sp., Ocimum basilicum, Ocimum sp., Opuntia sp., Origanum majorana,
Origanum sp., Origanum vulgare, Phaseolus sp., Phaseolus vulgaris, Pimenta sp., Pisum sativum, Pisum sativum var. macrocarpon,
Pisum sp., Pithecellobium dulce, Poaceae, Polianthes tuberosa, Porophyllum sp., Portulaca oleracea, Pyrus communis,
Raphanus sativus, Rosmarinus officinalis, Rubus sp., Saccharum officinarum, Salvia officinalis, Salvia sclarea, Salvia sp., Scabiosa sp.,
Solanaceae, Solanum integrifolium, Solanum lycopersicum var lycopersicum, Solanum melongena, Solanum sp., Solidago sp., Spinacia sp., Spiraea sp.,
Spondias purpurea, Suaeda sp., Tagetes erecta, Tagetes sp., Thymus sp., Thymus vulgaris, Vicia faba, Vigna sp., Yucca elephantipes, Zea mays,
Zea sp., Zingiber sp.
There are several records in PestID of H. zea from Mexico on Physalis (Physalis ixocarpa, Physalis philadelphica, Physalis pubescens),
some of these possibly represent interceptions of C. subflexa.
NOCTUIDAE - Helicoverpa zea (Boddie) *Non-Rep*
Setal map
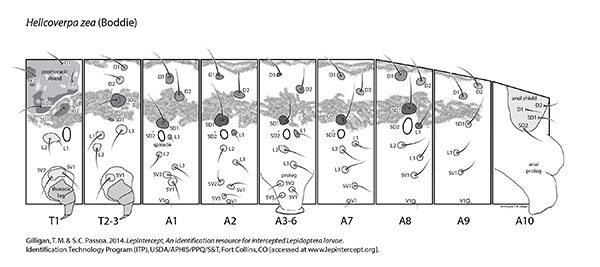 Helicoverpa zea setal map Helicoverpa zea setal map
<< Previous fact sheet Next fact sheet >>
 Fig. 1: Late instar, lateral view
Fig. 1: Late instar, lateral view |
 Fig. 2: Late instar, lateral view
Fig. 2: Late instar, lateral view |
 Fig. 3: Mid-instar, lateral view
Fig. 3: Mid-instar, lateral view |
 Fig. 4: Late instar, dorsal view
Fig. 4: Late instar, dorsal view |
 Fig. 5: Late instar, dorsal view
Fig. 5: Late instar, dorsal view |
 Fig. 6: Late instar, dorsal view
Fig. 1: Late dorsal, lateral view |
 Fig. 7: Mid-instar, thorax
Fig. 7: Mid-instar, thorax |
 Fig. 8: A8 pinacula; arrows denote lack of microspines
Fig. 8: A8 pinacula |
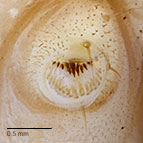
Fig. 9: Crochets |
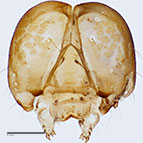
Fig. 10: Head |
 Fig. 11: Hypopharyngeal complex, lateral view
Fig. 11: Hypo. complex |
 Fig. 12: Hypopharyngeal complex, dorsal view
Fig. 12: Hypopharyngeal complex, dorsal view |

Fig. 13: Mandible |

Fig. 14: Mandible |
|
|
|
|
|