CRAMBIDAE - Diaphania hyalinata-indica complex
Taxonomy
Pyraloidea: Crambidae: Spilomelinae: Diaphania hyalinata-indica complex
Common names: melonworm, cucumber moth, pumpkin caterpillar
Synonyms: [too many to list - see http://www.pyraloidea.org for complete taxonomy]
Larval diagnosis (Summary)
- No genal spot
- Outer tooth on the mandible
- Bisetose SV group on A1
- Abdominal crochets in a mesal penellipse
- Must be on cucurbits if D. hyalinata; D. indica has a wider host range but is often on Momordica;
status of D. hyalinata on Momordica is unknown
Host/origin information
Larvae in the D. hyalinata-indica complex are most frequently intercepted from Mexico (33%) and the Caribbean (>60%). Common host/origin combinations are listed
below:
| Origin |
Host(s) |
| Dominican Republic | Luffa, Momordica |
| Haiti | Momordica |
| Jamaica | Momordica |
| Mexico | Cucurbita, Sechium |
Recorded distribution
Diaphania hylinata occurs from Canada to Argentina. Diaphania indica occurs in Florida, tropical America, and the Old World tropics.
Identification authority (Summary)
Identification of larvae in the D. hyalinata-indica complex is based on the absence of a genal spot, presence of an outer tooth on the mandible, and the
bisetose SV group on A1. None of these characters are very distinctive and numerous sibling species of Diaphania exist with unknown larvae.
These uncertainties all suggest a need for caution; see the Detailed Information tab.
Pest characterization
(Based on Cavey 2001, Whittle and Ferguson 1987)
- Taxonomy: Medium. At least genus identification is usually possible.
- Distribution: Medium. Diaphania indica is present in Florida but is not widespread in the rest of the United States.
Diaphania hyalinata is common wherever its hosts are grown. When one species is present in the U.S., but other species are not or
of limited distribution, Cavey (2001) suggests using Medium for this situation.
- Potential Impact: High. Both species are pests.
This ranking characterizes the D. hyalinata-indica complex as quarantine significant for the U.S.
CRAMBIDAE - Diaphania hyalinata-indica complex
Larval diagnosis (Detailed)
Diaphania indica and D. hyalinata were placed in the D. hyalinata species group by Clavijo (1990); later called the D. indica
complex by Solis (2011). These two species are treated together because the larvae are difficult to separate and there is evidence
that both are intercepted at United States ports. There are other species of Diaphania in Latin America that are similar to both
D. indica and D. hyalinata in adult coloration (Arias and Clavijo 2001) and potentially part of this complex, but at present these
other species do not appear to be pest species of concern to APHIS.
The larva of D. hyalinata, called the melonworm, was partially described by Smith (1911), Peterson (1962), Weisman (1986),
Neunzig (1987), Solis (2011), and Schnitzler et al. (2011). Color photographs were published by King and Saunders (1984),
Passoa (1985), Schmutterer et al. (1990), Sparks and Liu (2001), and Cranshaw (2004).
The larva of D. indica, called the pumpkin caterpillar (Whittle and Ferguson 1987), was described by Mathur and Singh (1963), Piao et al. (2008),
and Singh (2012). Color photographs can be found in NBAII (2013). The life cycle was illustrated by Singh (2012).
Typically, larvae of the D. hyalinata-indica complex have no genal spot, an outer tooth on the mandible, a bisetose SV group
on A1, and abdominal crochets in a mesal penellipse. The L1 seta on A8, at least in D. hyalinata, is separated from the spiracle
by the vertical diameter of the spiracle (Peterson 1962: L51).
Larvae of the D. hyalinata-indica complex are most likely to be confused with D. nitidalis. Unlike D. hyalinata and D. indica,
D. nitidalis lacks an outer tooth on the mandible above the lateral setae and has a genal spot (see D. nitidalis fact sheet).
There are also coloration differences between the two taxa (Smith 1911, Passoa 1985, Weisman 1986, Whittle and Ferguson 1987,
Neunzig 1987, Solis 2011). Live larvae of D. nitidalis are green, becoming copper to pink at maturity, without the white subdorsal
longitudinal stripes found on D. hyalinata and D. indica. Another difference is that early instars of D. nitidalis have dark
pigmented body pinacula. All instars of larvae in the D. hyalinata-indica complex have pale pinacula, or at most two tiny black
spots on the subdorsal pinaculum of the mesothorax and metathorax, and do not turn pink or copper before pupation. Of course the
white subdorsal lines usually fade in preserved specimens. Sometimes the mandibles of D. hyalinata show through the transparent
head before molting to superficially give the impression of a genal spot which could cause the larva to be wrongly identified as
D. nitidalis.
According to Capps (1948), some N. elegantalis have a bisetose SV group on A1. These could potentially be confused with larvae of
the D. hyalinata-indica complex. Among many differences, the mandible of the D. hyalinata-indica complex has an outer tooth lacking
in N. elegantalis.
So far no characters have been found to separate D. indica from D. hyalinata although the position of the substemmatal and
stemmatal setae may differ (Passoa 1985). SS2 and SS3 are spaced closer than SS3 and S3 in D. indica. The distance between SS2
and SS3 is subequal to that between SS3 and S3 in D. hyalinata. The drawing in Singh (2012) seems to fit this diagnosis but more
material is needed to evaluate the variability of this character. The larval antenna of D. indica was described in CEIR (1958),
but no comparisons were made to other species. Mathur and Singh (1963) used the height of the adfrontal area and position of AF1
to separate D. indica from other Diaphania species in India. This, and the pattern of pigmentation on the prothoracic and anal
shields they illustrated (Mathur and Singh (1963: fig. 36, 37), potentially can help identify D. indica. Nevertheless, accurate
morphological identifications are easily made by rearing because the genitalia of both sexes and pupal morphology are very
different in D. indica and D. hyalinata.
Passoa (1985: figs. 173, 174) compared the pupal heads of D. indica and D. hyalinata. The pilifers of D. hyalinata are much
longer than broad giving the head a long and slender appearance. The opposite is true for D. indica; the pilifers are broader
than long causing the head to appear shorter and stouter. This difference is often visible on cast skins. Whittle and Ferguson (1987)
added that maxillae extend to A8 or A9 in D. hyalinata, but only to A7 in D. indica. The pupal abdominal dorsum of D. nitidalis
is roughened; this separates it from D. hyalinata (Passoa 1985: figs. 170, 171) and D. indica which have punctate abdomens.
Identification authority (Detailed)
Identification of larvae in the D. hyalinata-indica complex is based on the absence of a genal spot, presence of an outer tooth on the mandible,
and the bisetose SV group on A1. None of these characters are very distinctive. Many species of crambids lack a genal spot. The
presence of an outer tooth on the mandible is unusual but a similar outer tooth is also found on Maruca vitrata, for example.
The bisetose SV group on A1 is helpful, but again not unique. A lack of characters for recognizing the D. hyalinata-indica complex
is compounded by numerous sibling species of Diaphania and relatives (in both the New and Old World) with unknown larvae.
Larvae of both the D. hyalinata-indica complex and D. nitidalis have a row of microspines at the base of the proleg but the distribution of this character in
Crambidae has not been studied. These uncertainties all suggest a need for caution. Unless the specimen is clearly in the in the D. hyalinata-indica
complex from the appropriate host, use Crambidae because no single character can separate the subfamilies Pyraustinae and Spilomelinae.
Origin and host are both helpful for identification of the D. hyalinata-indica complex. Diaphania indica occurs in Florida,
tropical America, and the Old World tropics (Clavijo 1990). Diaphania hylinata occurs from Canada to Argentina (Solis 2011).
Therefore, Old World interceptions of the D. hyalinata-indica complex, especially on crops, are D. indica and not D. hyalinata.
The hosts of D. hyalinata are in the Cucurbitaceae, the one exception on Ipomea (Clavijo 1990) is probably it atypical.
Diaphania indica has a wider host range that includes Cucurbitaceae, legumes, cotton (Clavijo 1990) and eggplant (CEIR 1958).
Thus, interceptions of the D. hyalinata-indica complex outside of the Curcubitaceae are D. indica and not D. hyalinata.
Larvae of the D. hyalinata-indica complex are most often foliage feeders, but do bore in fruit, stems and flowers, although
this is not their typical biology (Clavijo 1990).
There is a need to clarify the relative abundance of the D. hyalinata-indica complex on Momordica at United States ports
and in the New World in general, especially in Florida (Clavijo 1990). One D. indica was reared from a larva intercepted
from Jamaica on Momordica (adult det. A. Solis) in 1995. Several larvae were collected at Homestead, Florida, in 1983 by
S. Passoa on Momordica. These were reared to the pupal stage and identified as D. indica by pupal characters. The Florida
State Collection of Arthropods has a large series of D. indica immatures from Momordica in the D. Habeck collection (for example A-725).
Diaphania indica has been intercepted in consignments of Momordica from Kenya and Surinam in 2005 by the National Plant Protection
Organization, the Netherlands (CSL 2005). Because of these records, it is tempting to say that D. hyalinata is very rare on Momordica and
therefore D. indica is the name to use for this host. A rearing program was suggested for D. indica at U. S. ports many years ago
(Passoa and Cavey 1992). It is only necessary to rear larvae to the pupal stage. Of course, modern methods like DNA barcoding would
allow for more rapid identification.
CRAMBIDAE - Diaphania hyalinata-indica complex
Origin records
Members of the Diaphania hyalinata-indica complex have been intercepted from the following locations:
Antigua and Barbuda, Barbados, Cayman Islands, China, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic,
Ecuador, El Salvador, Ghana, Grenada, Guatemala, Guyana, Haiti, Honduras, India, Israel,
Jamaica, Liberia, Mexico, Morocco, Nigeria, Palestinian Territory, Puerto Rico, Somalia, St. Lucia,
St. Maarten, Trinidad and Tobago, Turks and Caicos Islands, US Virgin Islands
Host records
Members of the Diaphania hyalinata-indica complex have been intercepted on the following hosts:
Abelmoschus esculentus, Abelmoschus sp., Alstroemeria sp., Amaranthus sp., Annona muricata*, Antirrhinum sp.,
Apium graveolens, Artocarpus altilis, Cajanus cajan, Capsicum frutescens, Carica papaya, Cercis sp.,
Chenopodium berlandieri ssp nuttalliae, Chenopodium sp., Chrysanthemum sp., Citrus sp.*, Coccinia grandis,
Coccinia sp., Coccoloba uvifera*, Cocos sp., Corchorus sp., Coriandrum sativum, Cucumis sativus, Cucumis sp.,
Cucurbita maxima, Cucurbita moschata, Cucurbita pepo, Cucurbita sp., Cucurbitaceae, Curcubita sp., Cymbopogon sp.,
Jatropha sp., Lagenaria siceraria, Limonium sp., Luffa acutangula, Luffa aegyptiaca, Luffa sp.,
Malvaceae, Mentha sp., Momordica balsamina, Momordica charantia,
Momordica indica, Momordica sp., Moringa citrifolia, Opuntia sp.*, Origanum sp., Pachyrhizus erosus,
Petroselinum crispum, Phaseolus sp., Physalis pubescens, Physalis sp., Phytolacca sp., Piper sp.,
Porophyllum ruderale, Portulaca oleracea, Salvia officinalis, Sechium edule, Sechium sp., Senna sp., Smilax sp.,
Solanum melongena*, Solanum sp.*, Solanum torvum*, Stachytarpheta jamaicensis, Thymus caespititius, Thymus vulgaris,
Trichosanthes sp., Xanthosoma brasiliense, Zea mays*
Although D. indica has a wide host range, records on Annona, citrus, seagrape, Opuntia, Solanum, and corn are among those needing
confirmation (indicated here with an *). The normal hosts of D. indica are in the Cucurbitaceae, Leguminosae, and Malvaceae (CSL 2005).
CRAMBIDAE - Diaphania hyalinata-indica complex
Setal map
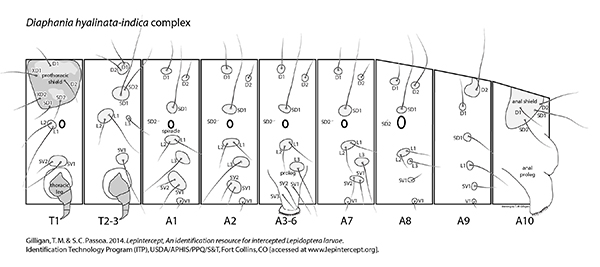 Diaphania hyalinata-indica complex setal map Diaphania hyalinata-indica complex setal map
<< Previous fact sheet Next fact sheet >>
 Fig. 1: Late instar, lateral view
Fig. 1: Late instar, lateral view |
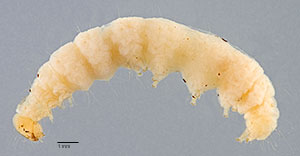 Fig. 2: Late instar, lateral view
Fig. 2: Late instar, lateral view |
 Fig. 3: Head and thorax, lateral view; note absence of genal spot
Fig. 3: Head and thorax, lateral view |
 Fig. 4: Bisetose SV group on A1
Fig. 4: SVs on A1 |
 Fig. 5: Abdominal crochets in a mesal penellipse
Fig. 5: Crochets |
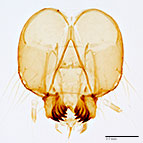
Fig. 6: Head |
 Fig. 7: Hypopharyngeal complex, lateral view
Fig. 7: Hypo. complex |
 Fig. 8: Mandible with outer tooth
Fig. 8: Mandible |
|
|
|
|
|
|