NOCTUIDAE - Mamestra brassicae (Linnaeus)
Taxonomy
Noctuoidea: Noctuidae: Noctuinae (s.l.): Mamestra brassicae (Linnaeus)
Common names: cabbage moth
Synonyms: Phalaena omicron, Noctua albidilinea, Hypobarathra unicolor
Larval diagnosis (Summary)
- Large retinaculum on the mandible
- SD1 and SD2 on the mesothorax and metathorax connected to a tonofibrillary platelet by a minute dark bar
- Black spots surround the abdominal spiracles
- Spinneret about four times as long as first segment of labial palpi
- Abdominal prolegs with a sclerotized shield
Host/origin information
More than 92% of the interception records for M. brassicae are from the Netherlands. The most common hosts are listed below:
| Origin |
Host(s) |
| Netherlands | Aconitum, Amaranthus, Brassica, Delphinium |
Recorded distribution
A Palearctic species, Mamestra brassicae is found from Europe to Japan and subtropical Asia (including India). It is also present in North Africa
(Canary Islands, Libya, and Morocco) (Carter 1984).
Identification authority (Summary)
Accurate identification of M. brassicae involves origin, morphology, and color pattern. Because this pest is polyphagous, the hostplant offers few
clues in spite of the common name. A positive identification can be made if the specimen possesses all of the morphological characters listed above (not just the proper mandible)
and originates from within the recorded distribution for this species.
Pest characterization
(Based on Cavey 2001, Carter 1984)
- Taxonomy: High. Species identification is possible in late instars.
- Distribution: High. Mamestra brassicae is not present in the U.S.
- Potential Impact: High. Mamestra brassicae is a serious pest.
This ranking characterizes Mamestra brassicae as quarantine significant for the U.S.
NOCTUIDAE - Mamestra brassicae (Linnaeus)
Larval diagnosis (Detailed)
Various instars of the cabbage moth, Mamestra brassicae, were described in detail by Sannino and Espinosa (1999). Other
morphological details were illustrated by Weisman (1986), Merzheevskaya (1988), Beck (1999-2000), and Ahola and Silvonen [2008].
Gomez de Aizpurua (2002), Porter (1997), Beck (1999-2000), and Ahola and Silvonen [2008] illustrated the larva in color.
Weisman (1986) used several characters to recognize M. brassicae. These included having a retinaculum (= basal process, inner tooth)
on the mandible and both SD setae of the mesothorax and metathorax connected to a tonofibrillary platelet (= muscle attachment) by a minute
dark bar. Beck (1999-2000), in his revision of European noctuid larvae, added the following morphological features: spinneret about four
times as long as first segment of labial palpi, sixteen stout spinelike blades present on the hypopharyngeal complex and abdominal prolegs
with a sclerotized shield. The mouthpart drawings in Ahola and Silvonen [2008: 570] agree with Beck's (1999) description. A long spinneret
was used by Merzheevskaya (1988) to separate M. brassicae from related species in the western Russian fauna. Sannino and Espinosa (1999)
called attention to a characteristic almost circular yellow spot between stemma one and stemma two. The dorsally grooved spinneret and
hairlike SD1 on A9 (Ahola and Silvonen [2008]) in M. brassicae is typical for the subfamily Noctuinae (s.l.) as defined in the broad sense to
include the Hadeninae (Lafontaine and Fibiger 2006).
Although the larva of M. brassicae is very variable in color and pattern (Beck 1999-2000), these characters are important. Whittle (1986)
and Gomez de Aizpurua (2002) illustrated two larval color forms of M. brassicae. Middle instars are green with a broad white spiracular
stripe that passes through the spiracles on A1-7 and below the much larger spiracle of A8. Mature larvae may or may not have dorsal markings
like a chevron. There is often a thin dark transverse line (cross bar) on A8 (Sannino and Espinosa 1999). Although
these features are characteristic of M. brassicae, they do occur on other noctuid genera as well. The most distinctive marking is a black
spot which surrounds the abdominal spiracles (Ahola and Silvonen [2008: 394]). This pattern is also found on
Lacanobia suasa (Merzheevskaya 1988, Porter 1997), but that species does not have a long spinneret or blades on the hypopharyngeal complex
(Beck 1999). One common North American species, Orthosia rubescens, has the spiracles surrounded by black markings (Wagner et al. 2011) in
a pattern similar to M. brassicae. However, the mouthparts of the two species are completely different (see Godfrey 1972).
The first instar of M. brassicae has pigmented body pinacula and a characteristic prothoracic shield pattern illustrated by
Sannino and Espinosa (1999). The outer margins of the shield are outlined in black enclosing two spots. The caption to Figure 4 of their
paper calls it a prothoracic shield - this is a misprint for anal shield.
Identification authority (Detailed)
Accurate identification of M. brassicae involves origin, morphology, and color pattern. Because this pest is polyphagous, the hostplant
offers few clues in spite of the common name.
Carter (1984) gave the distribution of M. brassicae as "throughout the Palearctic Region from Europe to Japan and into subtropical Asia,
including India." There is a map which shows two African localities, Libya and the Canary Islands in Whittle (1986). From time to time,
identifiers submit presumed M. brassicae specimens from central Africa. This is slightly, but significantly, outside the known range of
this pest. Because the climate and plants are different in the northern and central regions of Africa, not all parts of Africa may be
ecologically suitable for M. brassicae; indeed, neither Forsyth (1966) in Ghana nor Schmutterer (1969) on pests of crops in central and
northeastern Africa include M. brassicae in their works. On the other hand, Whittle (1986) suggests that records from Greece, Japan, and
Morocco for M. brassicae are errors representing transshipments. There is nothing unusual about any of these three countries given the
above synopsis of the known distribution. The best solution is to restrict M. brassicae identifications to origins consistent with the
known distribution of the pest. Of course, New World records for M. brassicae are suspect unless there is evidence of transshipment
or other similar circumstance.
In the past, identifiers using Weisman (1986) often stopped at M. brassicae after seeing the proper mandible without paying attention
to other characters or clues. It is important to understand that a retinaculum is present in hundreds of species of Noctuidae or Erebidae
worldwide. Even the minute SD bars are common in many related species. Moreover, it is not clear which instars of M. brassicae have the
retinaculum or if it can be worn off (see fact sheet on H. virescens).
Although Beck (1999-2000) and Ahola and Silvonen (2005, [2008]) often give long detailed color descriptions in their keys, most of
these are rarely useful to PPQ because all larvae are killed before submission to the port identifier. This results in specimens with
faded or lost colors. In some groups, given the ease of taking digital photos, it might be better to submit color photos with suspects
of key program pests. Mamestra brassicae would be a good example of this need; identifications are much less accurate on faded preserved
specimens. Specimens without any pattern or color should be left as "Noctuinae complex" if SD1 is hairlike on A9.
NOCTUIDAE - Mamestra brassicae (Linnaeus)
Origin records
Mamestra brassicae has been intercepted from the following locations:
Bosnia and Herzegovina, China, France, Israel, Italy, Japan, Lebanon, Mongolia,
Morocco, Netherlands, Portugal, South Korea, Spain
Locations from outside of the known distribution for this species likley represent misidentifications and are not listed here.
Host records
Mamestra brassicae has been intercepted on the following hosts:
Achillea sp., Aconitum napellus, Aconitum sp., Ageratum sp., Alchemilla sp., Allium porrum, Alocasia sp.,
Alstroemeria sp., Amaranthus caudatus, Amaranthus sp., Ammi majus, Anemone coronaria, Anemone sp., Antirrhinum majus,
Apium graveolens, Asclepias sp., Asclepias tuberosa, Astilbe sp., Astrantia sp., Brassaia sp.,
Brassica oleracea, Brassica oleracea var. botrytis, Brassica pekinensis, Brassica sp., Bupleurum griffithii,
Bupleurum sp., Calendula sp., Callistephus sp., Campanula sp., Capsicum annuum, Capsicum sp., Celosia sp.,
Chenopodium sp., Chrysanthemum sp., Cichorium sp., Consolida ajacis, Cotinus sp., Crocosmia sp., Cucumis sp.,
Curcubita sp., Cymbidium sp., Dahlia sp., Daucus sp., Delphinium sp., Deutzia sp., Dianthus sp., Dryopteris sp.,
Eryngium alpinum, Eryngium sp., Eupatorium sp., Eustoma grandiflorum, Eustoma sp., Freesia sp., Gentiana sp.,
Gloriosa sp., Helenium sp., Helianthus sp., Helleborus sp., Hydrangea sp., Hypericum sp., Lactuca sativa, Lamiaceae,
Lathyrus sp., Leonotis sp., Leucospermum sp., Lilium sp., Lisianthus sp., Lycopersicon sp., Lysimachia sp., Mentha sp.,
Origanum vulgare, Paeonia lactiflora, Paeonia sp., Phlox sp., Platycodon sp., Ranunculus sp., Rosa sp.,
Rudbeckia sp., Scabiosa sp., Setaria italica, Solanum sp., Solidago sp., Syringa sp., Thymus vulgaris,
Trachelium sp., Veronica sp., Viburnum opulus, Viburnum sp.
NOCTUIDAE - Mamestra brassicae (Linnaeus)
Setal map
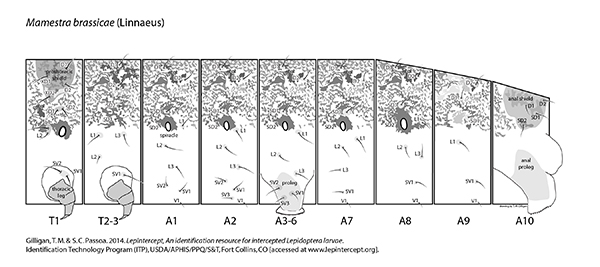 Mamestra brassicae setal map Mamestra brassicae setal map
<< Previous fact sheet Next fact sheet >>
 Fig. 1: Late instar, lateral view
Fig. 1: Late instar, lateral view |
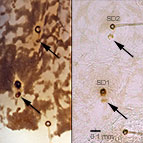 Fig. 2: SD1 and SD2 on T2
Fig. 2: SD1-2 on T2 |
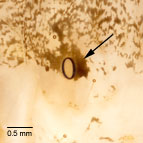 Fig. 3: Coloration surrounding spiracle
Fig. 3: Spiracle |
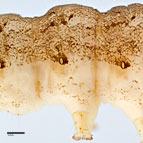 Fig. 4: Detail of abdominal segment A3
Fig. 4: A3 with proleg |
 Fig. 5: Abdominal proleg; arrow denotes sclerotized shield
Fig. 5: Proleg |

Fig. 6: Crochets |
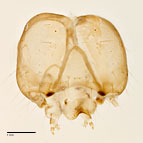
Fig. 7: Head |
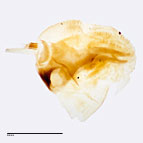 Fig. 8: Hypopharyngeal complex, lateral view
Fig. 8: Hypo. complex |
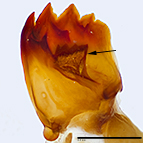 Fig. 9: Mandible; arrow denotes large retinaculum
Fig. 9: Mandible |
|
|
|
|
|